One-Minute Deep Breathing Assessment and its Relationship to 24-h Heart Rate Variability Measurements
Rollin McCraty, Mike Atkinson, Joe Dispenza1 | 01 October 2019
Abstract
Heart rate variability (HRV), the change in the time intervals between adjacent heartbeats, is an emergent property of interdependent regulatory systems that operates on different time scales to adapt to environmental and psychological challenges. Low age-adjusted HRV has also been confirmed as a strong, independent predictor of future health problems in both healthy people and in patients with a wide range of diseases and correlates with all-cause mortality. 24-hour HRV recordings are considered the “gold standard” and have greater predictive power on health risk than short-term recordings. However, it is not always practical or cost effective to obtain 24-hour HRV recordings, and short-term recordings have been widely used in research and clinical applications for many years.
This study examined the correlations between a 10-minute resting state period, a 1-minute paced deep breathing protocol, response to handgrip and 24-hour HRV measures in 28 healthy individuals. Based on the results of the initial study, the primary study examined the correlations between the 1-minute paced deep breathing assessment and 24-hour measures in a general population of 805 individuals. Overall, the findings from the studies suggested that the 1-minute paced deep breathing assessment had high correlations with the 24-hour measures of vagally mediated HRV, and with VLF power.
The findings from this study suggest that the 1-minute paced deep breathing protocol is an ideal short-term assessment that can be used in a health risk screening context. When low values are observed, it is recommended that a 24-hour assessment be conducted.
Key words: HRV, Heart Rate Variability, Risk Assessment, Deep Breathing
Introduction
The investigation of the heart’s complex rhythms or what is now called heart rate variability (HRV) (Donald H. Singer et al., 1988) has rapidly expanded in more recent years. The beat-to-beat fluctuations in heart rate result from complex, nonlinear interactions among a number of different physiological systems. HRV is thus considered a measure of neurocardiac function that reflects heart–brain interactions and autonomic nervous system (ANS) dynamics (R. McCraty, Atkinson, Tomasino, & Bradley, 2009; F. Shaffer, McCraty, & Zerr, 2014). An optimal level of HRV reflects healthy function and an inherent self-regulatory capacity, adaptability, or resilience (F. C. Geisler, Kubiak, Siewert, & Weber, 2013; R. McCraty et al., 2009; R. McCraty, Childre, D, 2010; R McCraty & Zayas, 2014; Reynard, Gevirtz, Berlow, Brown, & Boutelle, 2011; Segerstrom & Nes, 2007; D. H. Singer, 2010) While too much instability, such as arrhythmias is detrimental to healthy functioning, too little variation indicates age-related system depletion, chronic stress, pathology, or inadequate functioning in various levels of self-regulatory control systems (Camm et al., 1996; Donald H. Singer et al., 1988; Thayer, Hansen, Saus-Rose, & Johnsen, 2009). It has clearly been shown that HRV declines with age and age-adjusted values should be used in the context of risk prediction (Umetani, Singer, McCraty, & Atkinson, 1998).
Reduced HRV was found to be a higher risk factor of death post-myocardial infarction than other known risk factors (Wolf, Varigos, Hunt, & Sloman, 1978), and can predict autonomic neuropathy in diabetic patients before the onset of symptoms (Braune & Geisendorfer, 1995; D. Ewing, Campbell, & Clarke, 1976; Vinik, Maser, Mitchell, & Freeman, 2003). Low age-adjusted HRV has also been confirmed as a strong, independent predictor of future health problems in both healthy people and in patients with a wide range of diseases and correlates with all-cause mortality (Dekker et al., 1997; Tsuji et al., 1994). A number of studies have shown that reduced HRV is associated with measures of inflammation in subjects with no apparent heart disease (Sajadieh et al., 2004). Reduced HRV is also observed in patients with autonomic dysfunction, anxiety, depression, asthma, and sudden infant death syndrome (Agelink, Boz, Ullrich, & Andrich, 2002; Carney et al., 2001; Cohen & Benjamin, 2006; Kazuma, Otsuka, Matsuoka, & Murata, 1997).
HRV also indicates psychological resiliency and behavioral flexibility, reflecting an individual’s capacity to self-regulate and effectively adapt to changing social or environmental demands (Beauchaine, 2001; Berntson, Norman, Hawkley, & Cacioppo, 2008). A growing number of studies has specifically linked vagally-mediated HRV to self-regulatory capacity, (F. Geisler & Kubiak, 2009; Reynard et al., 2011; Segerstrom & Nes, 2007) emotional regulation, (Appelhans & Luecken, 2006; F. Geisler, Vennewald, Kubiak, & Weber, 2010) social interactions, (F. C. Geisler et al., 2013; Smith et al., 2011) one’s sense of coherence (Nasermoaddeli, Sekine, & Kagamimori, 2004) and the personality character traits of Self-Directedness (Zohar, Cloninger, & McCraty, 2013) and coping styles (Ramaekers, Ector, Demyttenaere, Rubens, & Van de Werf, 1998).
Several studies have shown an association between higher levels of vagally-mediated resting state HRV and cognitive performance on tasks requiring the use of executive functions (Thayer et al., 2009). Thayer has shown that vagally-mediated HRV is correlated with prefrontal cortical performance and the ability to inhibit unwanted memories and intrusive thoughts, and that the prefrontal cortex can be taken “offline” when individuals are stressed or threatened. Thayer also has shown that prolonged prefrontal inactivity can lead to hypervigilance, defensiveness, and social isolation (Thayer et al., 2009).
HRV Analysis
The amount of HRV an individual has can be assessed with various analytical approaches, although the most commonly used are frequency domain (power spectral density) analysis and time domain analysis. The interactions between autonomic neural activity, blood pressure, respiratory and higher level control systems and environmental factors produce both short and longer-term rhythms in HRV measurements (Alabdulgader et al., 2018; 1996; Hirsch & Bishop, 1981; R McCraty et al., 2017; R. McCraty et al., 2009). As there are a number of recent reviews regarding the physiological mechanisms and interpretation of HRV, only a brief summary will be provided here (Ernst, 2017; Fatisson, Oswald, & Lalonde, 2016; Laborde, Mosley, & Thayer, 2017; F. Shaffer et al., 2014).
Frequency Domain Measurements
The main advantage of spectral analysis is that it supplies both frequency and amplitude information on the specific rhythms that exist in the HRV waveform, providing a means to quantify these oscillations over any given period. The international Task Force standardized the heart rhythm oscillations into four primary frequency bands: high-frequency (HF), low-frequency (LF), very-low-frequency (VLF), and ultra-low-frequency (ULF) (Camm et al., 1996). The values are expressed as the Power Spectral Density, which is the area under the curve (peak) in a given bandwidth of the spectrum (R McCraty & Shaffer, 2015).
The HF range is from 0.15 Hz to 0.4 Hz, which equates to rhythms with periods that occur between 2.5 and 7 seconds. This band reflects parasympathetic or vagal activity and is frequently called the respiratory band because it corresponds to the heart rate (HR) variations related to the respiratory cycle known as respiratory sinus arrhythmia.
The LF range is between 0.04 and 0.15 Hz, which equates to rhythms or modulations with periods that occur between 7 and 25 seconds. This region primarily reflects baroreceptor activity while at rest (Alberto Malliani, 1995). In ambulatory 24-hour HRV recordings, it has been suggested that the LF band reflects sympathetic activity and the LF/HF ratio has been controversially used to assess the balance between sympathetic and parasympathetic activity (A. Malliani, Lombardi, Pagani, & Cerutti, 1994; Pagani, Lombardi, & Guzzette, 1986; Pal et al., 2013). However, a number of researchers have challenged this perspective and have persuasively argued that in resting conditions, the LF band only reflects baroreflex activity and not cardiac sympathetic innervation.40, 71, 96, 105-107 In long-term ambulatory recordings, the LF band fairly approximates sympathetic activity when increased sympathetic activity occurs (Axelrod, Lishner, Oz, & al, 1987). However, this interpretation is inappropriate when short-term resting recordings are utilized (R McCraty & Shaffer, 2015).
The VLF is the power in the range between 0.0033 and 0.04 Hz, which equates to rhythms or modulations with periods that occur between 25 and 300 seconds. Although all 24-hour measures of HRV reflecting low HRV are linked with increased risk of adverse outcomes, the VLF band has stronger associations with all-cause mortality than the LF and HF bands (Hadase et al., 2004; Schmidt et al., 2005; Tsuji et al., 1996; Tsuji et al., 1994). Experimental evidence suggests that the VLF rhythm is intrinsically generated by the heart’s intrinsic cardiac nervous system and that the amplitude and frequency of these oscillations are modulated by efferent sympathetic activity (R McCraty & Shaffer, 2015).
The ULF range falls below 0.0033 Hz (333 seconds or 5.6 minutes). The circadian oscillations in heart rate is the primary source of this rhythm, although other very slow-acting regulatory processes add to the power in this band (Camm et al., 1996).
Time Domain Measurements
Time domain indices quantify the amount of variance in the inter-beat-intervals (IBI) using statistical measures. The three most commonly reported time domain measures are the SDNN, the SDNN index, and the RMSSD. The SDNN is the standard deviation of the normal-to-normal (NN) sinus-initiated inter-beat-intervals measured in milliseconds. This measure reflects the ebb and flow of all the factors that contribute to HRV. In 24-hour recordings, the SDNN is highly correlated with ULF and total power (Umetani et al., 1998). In short-term resting recordings, the primary source of the variation is vagally-mediated. The SDNN index is the mean of the standard deviations of all the NN intervals for each 5-minute segment. Therefore, this measurement only estimates variability due to the factors affecting HRV within a 5-minute period. In 24-hour HRV recordings, it is calculated by first dividing the 24-hour record into 288 five-minute segments and then calculating the standard deviation of all NN intervals contained within each segment. The SDNN Index is the average of these 288 values (Camm et al., 1996). This measure tends to correlate with VLF power over a 24-hour period (F. Shaffer et al., 2014).
The RMSSD is the root mean square of successive differences between normal heartbeats. This value is obtained by first calculating each successive time difference between heartbeats in milliseconds. Then, each of the values are squared and the result is the square root of the average of all squared successive difference. The RMSSD reflects the beat-to-beat variance in heart rate and is the primary time domain measure used to estimate the vagally-mediated changes reflected in HRV (Camm et al., 1996). The RMSSD is correlated with HF power (F. Shaffer et al., 2014).
Mean heart rate range (MHRR) is calculated by averaging the differences between maximum heart rate during inspiration and the minimum heart rate during expiration for each breathing cycle over the 1-minute test duration, typically 5-6 breaths. Mean inter-beat-interval range (MIBIR) is calculated the same as MHHR only using inter-beat intervals in milliseconds. This avoids the potential influence of the rate transformation to beats-per-minute used in the calculation of MHHR.
The expiratory-to-inspiratory ratio (E:I ratio), is the ratio of the longest R-R interval during expiration to the shortest R-R interval during inspiration. The mean of the ratios for each breathing cycle over the 1-minute test duration was used in this study.
Recording Lengths
HRV recording lengths can be obtained over periods that range from 1-minute to weeks, although the most common short-term recording length is 5-minutes, while the most common long-term period is 24-hours. The length of the recording period significantly affects HRV values (Laborde et al., 2017) and it is inappropriate to compare any HRV metrics when they are obtained from different recording lengths (Fred Shaffer & Ginsberg, 2017). In addition, the context in which the recording is made also significantly affects the values, such as resting state or ambulatory, seated or supine. 24-hour HRV recordings should be obtained to provide comprehensive assessment of VL F and ULF fluctuations (Kleiger, Stein, & Bigger, 2005).
Obviously, longer recording periods provide more information regarding autonomic function, health status, stress reactions, and environmental influences than is possible in short-term recordings. For example, 24-hour heart rate (HR), responses to stressors, workloads, and different aspects of circadian rhythms, differences in day-night HR, sleep-wake cycles, dream activity, etc. can only be observed in 24-hour recordings. Thus, 24-hour HRV recordings are considered the “gold standard” for clinical HRV assessment (Fred Shaffer & Ginsberg, 2017) and have greater predictive power or health risk than short-term recordings (L. Fei, X. Copie, M. Malik, & A. J. Camm, 1996; Kleiger et al., 2005; Nolan et al., 1998), which typically do not correlate well with 24-hour recordings (Lü Fei, Xavier Copie, Marek Malik, & A John Camm, 1996).
Of course, it is not always practical or cost effective to obtain 24-hour HRV recordings in research, clinical, mental health or large-scale health risk assessment contexts. Therefore, short-term recordings have been widely used in research for many years (Camm et al., 1996) and more recently in consumer applications. It should be kept in mind that in short-term resting recordings, the primary source of the variation is due to vagally-mediated (parasympathetic) processes (F. Shaffer et al., 2014).
While the most common short-term recording protocol is for 5-minutes in a seated resting state (Camm et al., 1996), researchers have utilized ultra-short recordings ranging from 10-seconds to 240-seconds (Baek, Cho, Cho, & Woo, 2015; Bradley et al., 2010; van den Berg et al., 2018). In a study that investigated the correlations between the standard 5-minute and ultra-short HRV recordings in a large population, it was found that different minimum recoding lengths were required for each HRV variable and age group. The basic findings were that HR required 10-secs, HF power required 20-secs, RMSSD required 30 secs, LF power required 90-secs, the SDNN required 240-seconds, while the VLF power required 270-seconds (Baek et al., 2015).
Another approach to short-term HRV assessments evolved from protocols developed for autonomic function assessments in diabetic patients called heart rate response to deep breathing (D. J. Ewing, Martin, Young, & Clarke, 1985; Watkins & MacKay, 1980). For this assessment the patient sits quietly and breathes deeply and evenly at a rate of 6 breaths per minute for three successive breathing cycles. The maximum and minimum heart rates during each breathing cycle are measured and expressed as the maximum and minimum differences in heart rate. This assessment was found to have a better diagnostic utility in a diabetic patients than the Valsalva maneuver, lying to standing heart rate response, postural blood pressure change and sustained handgrip test (D. J. Ewing et al., 1985). It was found that using a 1-minute paced deep breathing at 6-breaths per minute protocol as a prognostic index after myocardial infarction as an assessment of HRV was a good predictor for all-cause mortality and sudden death in this population (Katz, Liberty, Porath, Ovsyshcher, & Prystowsky, 1999). Therefore, it is considered one of the most reliable tests of cardio-vagal function (Low, 2004).
The two most widely used metrics for deep breathing assessment are the mean heart rate range (MHRR) and the expiratory-to-inspiratory ratio (E:I). The MHRR method is measured from a series of successive deep breaths, at a rate of 6 breaths per minute. The difference between the maximum and minimum heart rate during each breathing cycle is calculated. The result is expressed as the mean of the heart rate differences in beats per minute (BPM) (Shields, 2009). The E:I ratio assesses the ratio of the longest R-R interval during expiration to the shortest R-R interval during inspiration (Ziegler et al., 1992). In essence, the 1-minute deep breathing assessment is a type of “challenge test” used to determine the maximum amount of vagally-mediated (parasympathetic) HRV their autonomic nervous system is capable of producing at the time of the measurement. In a study with 293 participants ranging in age from 10 to 82 years, the 5-minute resting HRV and 1-minute paced deep breathing assessment were compared on both time and frequency domain measures and the MHHR and E:I ratios. It was found that the maximum variation of heart rate measures in the 1-minute deep breathing test had the highest negative correlations with age as compared to all the HRV parameters in the 5-minute resting assessment (Russoniello, Zhirnov, Pougatchev, & Gribkov, 2013).
We are only aware of one study that has examined the correlations between short-term and 24-hour measures of HRV, which was conducted in a population of patients with confirmed myocardial infarction. The correlation between a 5-minute resting state recording and 24-hour measures was relatively poor (r=0.51), although it was significant. At one-year follow-up, both the short and long-term measures were significantly lower in patients who died than in survivors. However, the long-term assessment was clearly superior to the resting state, short-term assessments in predicting risk. The authors suggested that short-term recordings should be used for all patients and a 24-hour assessment be conducted in people with depressed short-term HRV values (Lü Fei et al., 1996).
Methods and procedures
In the studies reported here, we examined the correlations between short-term resting state HRV measures, the 1-minute paced deep breathing assessment and 24-hour measures. Two studies were conducted. The first was a smaller laboratory based pilot study (N-28) with healthy individuals that compared a 10-minute resting state period, one-minute resting states (one-min average of the 10-minute recording), HRV response to handgrip exercise, the 1-minute paced deep breathing assessment, and 24-hour measures. The second primary study examined the correlations between the 1-minute paced deep breathing assessment and 24-hour measures in a general population (N=805) of individuals, independent of health status.
Participants
The participants in the pilot study (N-28) were healthy volunteers who were employees of one of the two HeartMath organizations located in Boulder Creek, CA. 70% were female (17 female, 11 male). The group as a whole had a mean age of 55, (range 25-64 years). Those with a known health disorder, or who took any medications known to affect autonomic function were excluded from the study. The study took place in the fall of 2010.
For the primary study, the participants (N=805) were recruited from a general population of individuals, independent of health status, who were attending one of a series of self-development conferences between 2014 and 2016 in various cities such as Cabo, Bon and Tacoma. 73% were female (596 female, 213 male). The mean age was 50.1 (range 19-89 years). There were no exclusion criteria, other than agreeing to sign the informed consent form. The research met all applicable standards for the ethics of experimentation in accordance with the Declaration of Helsinki. All participants signed informed consent and were free to withdraw from the study at any time.
HRV Data Collection
All participants in both studies underwent 24-hour ambulatory HRV recordings (Bodyguard2, Firstbeat Technologies Ltd., Jyväskylä, Finland). Participants were instructed how to stop the recorder at the end of the 24-hour recording period. Ambu Blue Sensor VL microporous breathable disposable electrodes were used for all of the recordings. The electrodes were placed in a modified V5 position. The HRV recorder calculates the RR Interval (R is a point corresponding to the peak of the QRS complex of the ECG wave; and RR is the interval between successive Rs) from the electrocardiogram sampled at 1000 Hz. The RR interval data were stored locally in the device memory, and downloaded to a computer workstation at the completion of the recordings.
All of the HRV recordings were analyzed using DADiSP 6.7. Inter-beat-intervals greater or less than 30% of the mean of the previous four intervals were considered artifacts, and were removed from the analysis record. Following an automated editing procedure, all of the recordings were manually reviewed by an experienced technician, and, if needed, corrected. Daily recordings were processed in consecutive 5-min segments in accordance with the standards established by the HRV Task Force.(Novak, Saul, & Eckberg, 1997) Any 5-minute segment with >10% of the IBIs either missing or removed in editing were excluded from the analysis.
Pilot Study
The participants completed a 10-minute resting state recording while seated upright in a comfortable chair, after which they did the 1-Minute HRV Deep Breathing Assessment, followed by a two-minute handgrip exercise shortly after the 24-Hour HRV recorder was connected. The ECG was recorded (Biopac MP 30) at a sample rate of 250 Hz during each segment of the protocol. For the resting state recording, the participants were instructed to sit quietly for 10-minutes without talking, chewing gum, reading, etc.), trying to remain as still as possible without sacrificing comfort. They were instructed not to meditate or use other similar practices and to not engage in intense mental or emotional activity and to keep their eyes open to help avoid falling asleep. For the 1-minute paced deep-breathing, the participants were instructed to breathe as deeply as they comfortably can at the rhythm shown on a breath-pacing screen (XXX) which was a ten-second rhythm (five seconds on the in-breath and five seconds on the out-breath). The pacing period lasted for one-minute (six respiration cycles). Some people needed a practice session before successfully completing the deep breathing aspect of the protocol. For the handgrip segment of the protocol, each participant’s maximal grip strength was first determined (Biopac MP3X dynamometer) from two brief contractions with their non-dominant hand. Subsequently, participants performed a sustained handgrip for 2-minutes at 35% of their maximal grip strength. This was typically an exhaustive exercise.
Primary Study
For the primary study, all participants were fitted with and wore an ambulatory HRV recorder for 24 hours. At the start of the recording period, participants were instructed in the 1-minute paced deep-breathing protocol as described above. The only difference was that the participants were not given a practice session.
Statistics
Correlation coefficients and P values were calculated for all 24 hour and short-term HRV measures (IBM SPSS ver 22). Correlations for the pilot study are presented in Table 1 and Correlations for the primary study are presented in Table 2.
Results
Pilot Study
As shown in Table 1, all the HRV assessments tested had significant, negative correlations with age. The highest correlations were with the 24-hour measures of LF and HF power (r = -0.62, -0.59 p <0.01) followed by Total power (TP) (r = -0.56 p <0.01), and VLF power (r = -0.48, p <0.05). The 1-minute deep breathing assessment had the next highest negative correlations: SDNN (r = -0.57, p <0.01), RMSSD (r = -0.56, p <0.01) and MHHR (r = -0.49, p <0.01). The correlations in the 10-minute resting state and handgrip assessment with age had similar results for HF power (r = -0.53, p <0.01). The LF power was (r = -0.41, p <0.01) for the 10-minute resting state, and (r = -0.46, p <0.01) for the handgrip assessment. The VLF power for the resting state was not significantly correlated with age.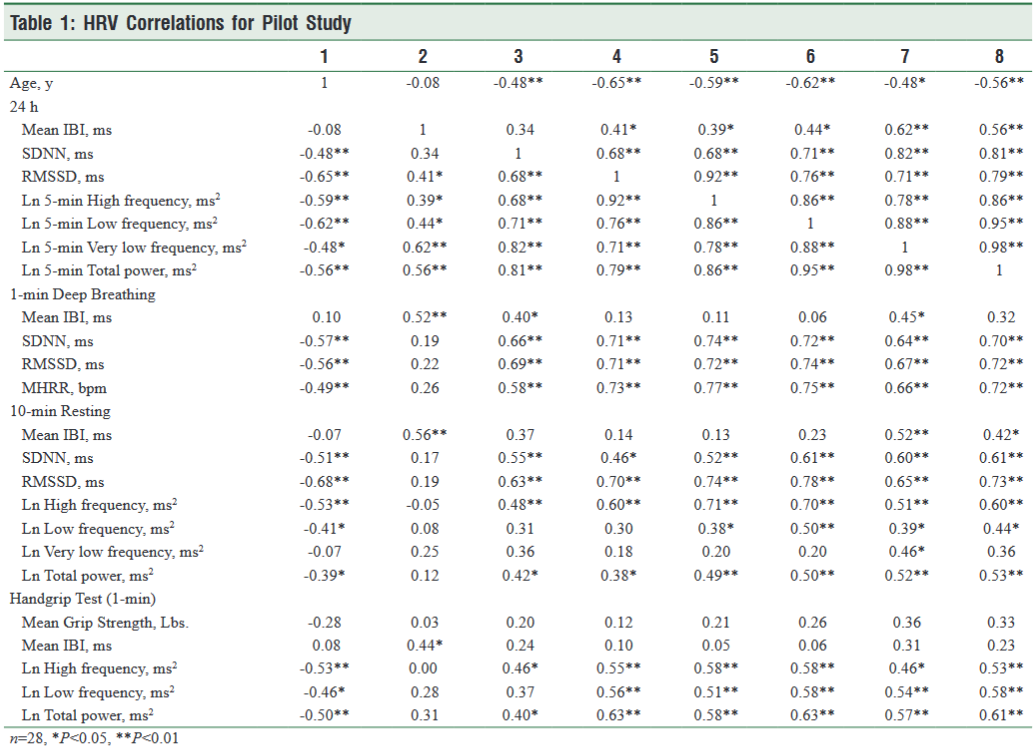
1-Minute Paced Deep Breathing
Overall, the 1-min. paced deep breathing had the highest correlations with the 24-hour measures. The SDNN was correlated with 24-hour HF power (r = 0.74, p <0.01), LF power (r = 0.72, p <0.01), VLF power (r = 0.64, p <0.01), TP (r = 0.70, p <0.01), RMSSD (r = 0.71, p <0.01), and SDNN (r = 0.66, p <0.01). Similarly, the RMSSD was correlated with HF power (r = 0.72, p <0.01), LF power (r = 0.74, p <0.01), VLF power (r = 0.67, p <0.01), TP (r = 0.72, p <0.01) and SDNN (r = 0.69, p <0.01). The MHHR was also highly correlated with HF power (r = 0.77, p <0.01), LF power (r = 0.75, p <0.01), VLF power (r = 0.66, p <0.01) and TP (r = 0.72, p <0.01), RMSSD (r = 0.73, p <0.01), and SDNN (r = 0.58, p <0.01).
10-Minute Resting State
In the 10-minute resting state assessment the HF power was correlated with 24-hour HF (r = 0.71, p <0.01), LF (r = 0.70, p <0.01), VLF (r = 0.51, p <0.01), TP (r = 0.60, p <0.01) RMSSD (r = 0.60, p <0.01), and SDNN (r = 0.48, p <0.01). LF power was correlated with 24-hour LF (r = 0.50, p <0.01), VLF power (r = 0.39, p <0.05), TP (r = 0.44, p <0.05) but was not correlated with the 24-hour RMSSD or SDNN. The only correlation of the VLF power in the resting state recording was with 24-hour VLF (r = 0.46, p <0.05). The TP was correlated with 24-hour HF (r = 0.49, p <0.01), LF (r = 0.50, p <0.01), VLF (r = 0.52, p <0.01), TP (r = 0.53, p <0.01) RMSSD (r = 0.38, p <0.05), and SDNN (r = 0.42, p <0.05).
Handgrip
During the handgrip assessment, the HF power was correlated with 24-hour HF (r = 0.58, p <0.01), LF (r = 0.58, p <0.01), VLF (r = 0.46, p <0.01), TP (r = 0.53, p <0.01), RMSSD (r = 0.55, p <0.01), and SDNN (r = 0.46, p <0.05). LF power was correlated with 24-hour HF (r = 0.51, p <0.01), LF (r = 0.58, p <0.01), VLF power (r = 0.54, p <0.05) TP (r = 0.58, p <0.05), and RMSSD (r = 0.56, p <0.01). The TP was correlated with 24-hour HF (r = 0.58, p <0.01), LF (r = 0.63, p <0.01), VLF (r = 0.72, p <0.01), TP (r = 0.61, p <0.01), RMSSD (r = 0.63, p <0.01), and SDNN (r = 0.40, p <0.05).
Based on the result of the pilot study, we chose to use the 1-min paced deep breathing protocol in the primary study.
Primary Study
All of the HRV assessments with the exception of IBIs in the 24-hour assessments had significant, negative correlations with age (Table 2). The highest correlations were with the LF (r = -0.521, p <0.01) and HF power (r = -0.506, p <0.01) followed by TP (r = -0.455 p <0.01), SDNN index (r = -0.436, p <0.01), RMSSD (r = -0.427, p <0.01), and VLF power (r = -0.377, p <0.05).
For the correlations between the 1-min. paced deep breathing assessment and 24-hour measures, the highest correlations were with mean IBIs (r = 0.761 p <0.01) and its related measure HR (0.756 p <0.01),. IBIs have an inverted relationship to heart rate where larger IBIs equated to a lower heart rate. Heart rate and IBIs are an ideal indicator of changes in the relative balance between parasympathetic and sympathetic activity and how the autonomic system responds and adapts to various types of stressors or challenges (R McCraty & Shaffer, 2015).
The highest correlations for the HRV variables were with the vagally mediated sources of HRV. The 1-min. paced deep breathing RMSSD was positively correlated with 24-hour HF power (r = 0.60, p <0.01), RMSSD (r = 0.62, p <0.01), LF power (r = 0.64, p <0.01). It was also correlated with VLF power (r = 0.57, p <0.01) TP (r = 0.42, p <0.01), SDNN index (r = 0.59, p <0.01), and SDNN (r = 0.41, p <0.01).
The MIBIR, ms was also highly correlated with the 24-hour vagally-mediated variables; HF power (r = 0.52, p <0.01), RMSSD (r = 0.52, p <0.01), and LF power (r = 0.58, p <0.01). It was also correlated with VLF power (r = 0.49, p <0.01), 5-min Total Power (r = 0.54, p < 0.01), TP (r = 0.37, p <0.01), SDNN index (r = 0.51, p <0.01), and SDNN (r = 0.36, p <0.01).
The 1-minute deep paced deep breathing SDNN was correlated with 24-hour HF power (r = 0.55, p <0.01), LF power r = (0.61, p <0.01), VLF power (r = 0.53, p <0.01), TP (r = 0.59, p <0.01), RMSSD (r = 0.55, p <0.01), SDNN index (r = 0.56, p <0.01), and SDNN (r = 0.40, p <0.01).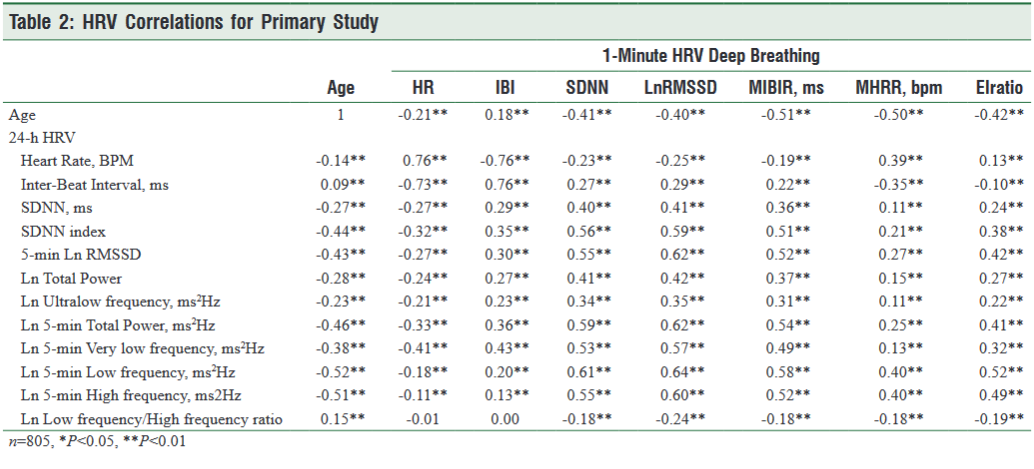
Discussion and Conclusions
We examined the correlations between HRV measures during a short-term resting state, 1-minute paced deep breathing, handgrip and 24-hour measures. In the pilot study, which was conducted in our laboratory with known healthy individuals, we able to insure that all the protocols were carefully followed. This was especially important for the 1-min paced deep breathing assessment, as it is important that the participants breathe as deeply as they comfortably can during the assessment. We found that many of the participants required a practice session before being able to get familiar with breathing as deeply as they comfortable could at the six breaths per minute rhythm.
In essence, the 1-minute paced deep breathing assessment determines the practical maximum HRV the cardiorespiratory system is capable of producing at the time of the assessment. This requires the participant to breathe at the resonant frequency of the cardiorespiratory system and to breathe as deeply as they comfortably can to maximize respiratory drive (Houtveen, Rietveld, & De Geus, 2002). Resonance occurs in an oscillatory system when there is a large sudden increase in amplitude at a specific frequency. Most mathematical models show that the resonance frequency of the human cardiovascular system is determined by the feedback loops between the heart and brain (Baselli et al., 1994; deBoer, Karemaker, & Strackee, 1987; Karavaev et al., 2016) and is approximately 0.1 Hz. Resonance is an aspect of the HRV coherence state, which is associated with a shift in autonomic balance toward increased parasympathetic activity, increased heart-brain synchronization, increased vascular resonance, and entrainment between diverse physiological oscillatory systems (R. McCraty et al., 2009; R. McCraty, Childre, D, 2010; Tiller, McCraty, & Atkinson, 1996).
Overall, the findings from the controlled pilot study suggest that the 1-min paced deep breathing assessment not only had the highest correlations with the 24-hour measures of vagally-mediated HRV, it also had slightly better correlations with VLF power than the 10-minute resting HRV.
The primary study was undertaken to increase generalizability of the pilot study’s findings with respect to the 1-min paced deep breathing assessment. While somewhat lower correlations to 24-hour measures of vagally-mediated HRV, and VLF power remained relevant, the RMSSD in 1-min. paced deep breathing assessment had a 0.60 correlation with 24-hour HF power, a 0.64 correlation with LF power and a 0.57 correlation with VLF power. This is an important factor as the low power in the VLF rhythm has stronger associations with all-cause mortality than the LF and HF bands (Tsuji et al., 1996; Tsuji et al., 1994), is associated with arrhythmic death (Bigger et al., 1992), PTSD (Shah et al., 2013), and high inflammation (Carney et al., 2007; Lampert et al., 2008). In addition, it was the shortest in time and is relatively easy to do.
In conclusion, the findings from this study suggest that the 1-minute paced deep breathing protocol is a useful and a potentially important test that can be used in health risk assessment context for screening patients. When low values are found, it is recommended that a 24-hour assessment be conducted.
References
Agelink, M. W., Boz, C., Ullrich, H., & Andrich, J. (2002). Relationship between major depression and heart rate variability. Clinical consequences and implications for antidepressive treatment. Psychiatry Res, 113(1-2), 139-149.
Alabdulgader, A., McCraty, R., Atkinson, M., Dobyns, Y., Stolc, V., A, V., & Ragulskis, M. ( 2018). Long-Term Study of Heart Rate Variability Responses to Changes in the Solar and Geomagnetic Environment. Nature Scientific Reviews, In Press
Appelhans, B., & Luecken, L. (2006). Heart Rate Variability as an Index of Regulated Emotional Responding. Review of General Psychology, 10(3), 229-240.
Axelrod, S., Lishner, M., Oz, O., & al, e. (1987). Spectral analysis of fluctuations in heart rate: An objective evaluation. Nephron, 45, 202-206.
Baek, H. J., Cho, C.-H., Cho, J., & Woo, J.-M. (2015). Reliability of ultra-short-term analysis as a surrogate of standard 5-min analysis of heart rate variability. Telemedicine and e-Health, 21(5), 404-414.
Baselli, G., Cerutti, S., Badilini, F., Biancardi, L., Porta, A., Pagani, M., . . . Malliani, A. (1994). Model for the assessment of heart period variability interactions of respiration influences. Medical and Biological Engineering and Computing, 32(2), 143-152.
Beauchaine, T. (2001). Vagal tone, development, and Gray's motivational theory: toward an integrated model of autonomic nervous system functioning in psychopathology. Dev Psychopathol, 13(2), 183-214.
Berntson, G. G., Norman, G. J., Hawkley, L. C., & Cacioppo, J. T. (2008). Cardiac autonomic balance versus cardiac regulatory capacity. Psychophysiology, 45(4), 643-652. doi:10.1111/j.1469-8986.2008.00652.x
Bigger, J. T., Jr., Fleiss, J. L., Steinman, R. C., Rolnitzky, L. M., Kleiger, R. E., & Rottman, J. N. (1992). Frequency domain measures of heart period variability and mortality after myocardial infarction. Circulation, 85(1), 164-171.
Bradley, R. T., McCraty, R., Atkinson, M., Tomasino, D., Daugherty, A., & Arguelles, L. (2010). Emotion self-regulation, psychophysiological coherence, and test anxiety: results from an experiment using electrophysiological measures. Appl Psychophysiol Biofeedback, 35(4), 261-283. doi:10.1007/s10484-010-9134-x
Braune, H. J., & Geisendorfer, U. (1995). Measurement of heart rate variations: influencing factors, normal values and diagnostic impact on diabetic autonomic neuropathy. Diabetes Res Clin Pract, 29(3), 179-187.
Camm, A. J., Malik, M., Bigger, J. T., Breithardt, G., Cerutti, S., Cohen, R. J., & Singer, D. H. (1996). Heart rate variability standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation, 93(5), 1043-1065.
Carney, R. M., Blumenthal, J. A., Stein, P. K., Watkins, L., Catellier, D., Berkman, L. F., . . . Freedland, K. E. (2001). Depression, heart rate variability, and acute myocardial infarction. Circulation, 104(17), 2024-2028.
Carney, R. M., Freedland, K. E., Stein, P. K., Miller, G. E., Steinmeyer, B., Rich, M. W., & Duntley, S. P. (2007). Heart rate variability and markers of inflammation and coagulation in depressed patients with coronary heart disease. J Psychosom Res, 62(4), 463-467. doi:10.1016/j.jpsychores.2006.12.004
Cohen, H., & Benjamin, J. (2006). Power spectrum analysis and cardiovascular morbidity in anxiety disorders. Auton Neurosci, 128(1-2), 1-8. doi:10.1016/j.autneu.2005.06.007
deBoer, R. W., Karemaker, J. M., & Strackee, J. (1987). Hemodynamic fluctuations and baroreflex sensitivity in humans: a beat-to-beat model. Am J Physiol, 253(3 Pt 2), H680-689.
Dekker, J. M., Schouten, E. G., Klootwijk, P., Pool, J., Swenne, C. A., & Kromhout, D. (1997). Heart rate variability from short electrocardiographic recordings predicts mortality from all causes in middle-aged and elderly men. The Zutphen Study. American Journal of Epidemiology, 145(10), 899-908.
Electrophysiology, T. F. o. t. E. S. o. C. a. t. N. A. S. o. P. a. (1996). Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Circulation, 93, 1043-1065.
Ernst, G. (2017). Heart-Rate Variability—More than Heart Beats? Frontiers in Public Health, 5, 240.
Ewing, D., Campbell, I., & Clarke, B. (1976). Mortality in diabetic autonomic neuropathy. Lancet, 1, 601-603.
Ewing, D. J., Martin, C. N., Young, R. J., & Clarke, B. F. (1985). The value of cardiovascular autonomic function tests: 10 years of experience in diabetes. Diabetes Care, 8, 491-498.
Fatisson, J., Oswald, V., & Lalonde, F. (2016). Influence diagram of physiological and environmental factors affecting heart rate variability: an extended literature overview. Heart Int, 11(1), e32.
Fei, L., Copie, X., Malik, M., & Camm, A. J. (1996). Short- and long-term assessment of heart rate variability for risk stratification after acute myocardial infarction. Am J Cardiol, 77(9), 681-684.
Fei, L., Copie, X., Malik, M., & Camm, A. J. (1996). Short-and long-term assessment of heart rate variability for risk stratification after acute myocardial infarction. American Journal of Cardiology, 77(9), 681-684.
Geisler, F., & Kubiak, T. (2009). Heart rate variability predicts self‐control in goal pursuit. European Journal of Personality, 23(8), 623-633.
Geisler, F., Vennewald, N., Kubiak, T., & Weber, H. (2010). The impact of heart rate variability on subjective well-being is mediated by emotion regulation. Personality and Individual Differences, 49(7), 723-728.
Geisler, F. C., Kubiak, T., Siewert, K., & Weber, H. (2013). Cardiac vagal tone is associated with social engagement and self-regulation. Biol Psychol, 93(2), 279-286. doi:10.1016/j.biopsycho.2013.02.013
Hadase, M., Azuma, A., Zen, K., Asada, S., Kawasaki, T., Kamitani, T., . . . Matsubara, H. (2004). Very low frequency power of heart rate variability is a powerful predictor of clinical prognosis in patients with congestive heart failure. Circ J, 68(4), 343-347.
Hirsch, J. A., & Bishop, B. (1981). Respiratory sinus arrhythmia in humans: How breathing pattern modulates heart rate. American Journal of Physiology, 241(4), H620-H629.
Houtveen, J. H., Rietveld, S., & De Geus, E. J. (2002). Contribution of tonic vagal modulation of heart rate, central respiratory drive, respiratory depth, and respiratory frequency to respiratory sinus arrhythmia during mental stress and physical exercise. Psychophysiology, 39(4), 427-436.
Karavaev, A. S., Ishbulatov, Y. M., Ponomarenko, V. I., Prokhorov, M. D., Gridnev, V. I., Bezruchko, B. P., & Kiselev, A. R. (2016). Model of human cardiovascular system with a loop of autonomic regulation of the mean arterial pressure. Journal of the American Society of Hypertension, 10(3), 235-243.
Katz, A., Liberty, I. F., Porath, A., Ovsyshcher, I., & Prystowsky, E. N. (1999). A simple bedside test of 1-minute heart rate variability during deep breathing as a prognostic index after myocardial infarction. American heart journal, 138(1), 32-38.
Kazuma, N., Otsuka, K., Matsuoka, I., & Murata, M. (1997). Heart rate variability during 24 hours in asthmatic children. Chronobiol Int, 14(6), 597-606.
Kleiger, R. E., Stein, P. K., & Bigger, J. T., Jr. (2005). Heart rate variability: measurement and clinical utility. Ann Noninvasive Electrocardiol, 10(1), 88-101. doi:10.1111/j.1542-474X.2005.10101.x
Laborde, S., Mosley, E., & Thayer, J. F. (2017). Heart rate variability and cardiac vagal tone in psychophysiological research–recommendations for experiment planning, data analysis, and data reporting. Front Psychol, 8, 213.
Lampert, R., Bremner, J. D., Su, S., Miller, A., Lee, F., Cheema, F., . . . Vaccarino, V. (2008). Decreased heart rate variability is associated with higher levels of inflammation in middle-aged men. Am Heart J, 156(4), 759 e751-757. doi:10.1016/j.ahj.2008.07.009
Low, P. A. (2004). Laboratory evaluation of autonomic function Supplements to Clinical neurophysiology (Vol. 57, pp. 358-368): Elsevier.
Malliani, A. (1995). Association of Heart Rate Variability components with physiological regulatory mechanisms. In M. Malik & A. J. Camm (Eds.), Heart Rate Variability (pp. 173-188). Armonk NY: Futura Publishing COmpany, Inc.
Malliani, A., Lombardi, F., Pagani, M., & Cerutti, S. (1994). Power spectral analysis of cardiovascular variability in patients at risk for sudden cardiac death. J Cardiovasc Electrophysiol, 5(3), 274-286.
McCraty, R., Atkinson, M., Stloc, V., Al Abdulgader, A., Vainoras, A., & Rangulas, M. (2017). Synchronization of Human Autonomic Nervous System Rhythms With Geomagnetic Activity in Human subjects Journal of Enviromental Research and Public Health, 14(770), 1-18. doi:10.3390/ijerph14070770
McCraty, R., Atkinson, M., Tomasino, D., & Bradley, R. (2009). The coherent heart: Heart-brain interactions, psychophysiological coherence, and the emergence of system-wide order. Integral Review, 5(2), 10-115.
McCraty, R., Childre, D. (2010). Coherence: Bridging Personal, Social and Global Health. Alternative Therapies in Health and Medicine, 16(4), 10-24.
McCraty, R., & Shaffer, F. (2015). Heart Rate Variability: New Perspectives on Physiological Mechanisms, Assessment of Self-regulatory Capacity, and Health Risk. Glob Adv Health Med, 4(1), 46-61. doi:10.7453/gahmj.2014.073
McCraty, R., & Zayas, M. (2014). Cardiac coherence, self-regulation, autonomic stability, and psychosocial well-being. Front Psychol, 5(September ), 1-13. doi:10.3389/fpsyg.2014.01090
Nasermoaddeli, A., Sekine, M., & Kagamimori, S. (2004). Association between sense of coherence and heart rate variability in healthy subjects. Environ Health Prev Med, 9(6), 272-274. doi:10.1007/BF02898142
Nolan, J., Batin, P. D., Andrews, R., Lindsay, S. J., Brooksby, P., Mullen, M., . . . Fox, K. A. (1998). Prospective study of heart rate variability and mortality in chronic heart failure: results of the United Kingdom heart failure evaluation and assessment of risk trial (UK-heart). Circulation, 98(15), 1510-1516.
Novak, V., Saul, J. P., & Eckberg, D. L. (1997). Task Force report on heart rate variability. Circulation, 96(3), 1056-1057.
Pagani, M., Lombardi, F., & Guzzette, S. (1986). Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circ Res, 59, 178-184.
Pal, G. K., Adithan, C., Ananthanarayanan, P. H., Pal, P., Nanda, N., Durgadevi, T., . . . Dutta, T. K. (2013). Sympathovagal imbalance contributes to prehypertension status and cardiovascular risks attributed by insulin resistance, inflammation, dyslipidemia and oxidative stress in first degree relatives of type 2 diabetics. PLoS One, 8(11), e78072. doi:10.1371/journal.pone.0078072
Ramaekers, D., Ector, H., Demyttenaere, K., Rubens, A., & Van de Werf, F. (1998). Association between cardiac autonomic function and coping style in healthy subjects. Pacing Clin Electrophysiol, 21(8), 1546-1552.
Reynard, A., Gevirtz, R., Berlow, R., Brown, M., & Boutelle, K. (2011). Heart rate variability as a marker of self-regulation. Appl Psychophysiol Biofeedback, 36(3), 209-215. doi:10.1007/s10484-011-9162-1
Russoniello, C. V., Zhirnov, Y. N., Pougatchev, V. I., & Gribkov, E. N. (2013). Heart rate variability and biological age: implications for health and gaming. Cyberpsychol Behav Soc Netw, 16(4), 302-308. doi:10.1089/cyber.2013.1505
Sajadieh, A., Nielsen, O. W., Rasmussen, V., Hein, H. O., Abedini, S., & Hansen, J. F. (2004). Increased heart rate and reduced heart-rate variability are associated with subclinical inflammation in middle-aged and elderly subjects with no apparent heart disease. Eur Heart J, 25(5), 363-370. doi:10.1016/j.ehj.2003.12.003
Schmidt, H., Muller-Werdan, U., Hoffmann, T., Francis, D. P., Piepoli, M. F., Rauchhaus, M., . . . Werdan, K. (2005). Autonomic dysfunction predicts mortality in patients with multiple organ dysfunction syndrome of different age groups. Crit Care Med, 33(9), 1994-2002.
Segerstrom, S. C., & Nes, L. S. (2007). Heart rate variability reflects self-regulatory strength, effort, and fatigue. Psychol Sci, 18(3), 275-281. doi:10.1111/j.1467-9280.2007.01888.x
Shaffer, F., & Ginsberg, J. (2017). An overview of heart rate variability metrics and norms. Frontiers in Public Health, 5, 258.
Shaffer, F., McCraty, R., & Zerr, C. (2014). A healthy heart is not a metronome: An integrative review of the heart's anatomy and heart rate variability. Front Psychol, 5:1040. doi:0.3389/fpsyg.2014.01040
Shah, A. J., Lampert, R., Goldberg, J., Veledar, E., Bremner, J. D., & Vaccarino, V. (2013). Posttraumatic stress disorder and impaired autonomic modulation in male twins. Biol Psychiatry, 73(11), 1103-1110. doi:10.1016/j.biopsych.2013.01.019
Shields, R. W., Jr. (2009). Heart rate variability with deep breathing as a clinical test of cardiovagal function. Cleve Clin J Med, 76 Suppl 2, S37-40.
Singer, D. H. (2010). High heart rate variability, marker of healthy longevity. Am J Cardiol, 106(6), 910.
Singer, D. H., Martin, G. J., Magid, N., Weiss, J. S., Schaas, J. W., Kehoe, R., . . . Lesch, M. (1988). Low heart rate variability and sudden cardiac death. Journal of Electrocardiology(Supplemental issue), S46-S55.
Smith, T. W., Cribbet, M. R., Nealey-Moore, J. B., Uchino, B. N., Williams, P. G., Mackenzie, J., & Thayer, J. F. (2011). Matters of the variable heart: respiratory sinus arrhythmia response to marital interaction and associations with marital quality. J Pers Soc Psychol, 100(1), 103-119. doi:10.1037/a0021136
Thayer, J. F., Hansen, A. L., Saus-Rose, E., & Johnsen, B. H. (2009). Heart rate variability, prefrontal neural function, and cognitive performance: the neurovisceral integration perspective on self-regulation, adaptation, and health. Ann Behav Med, 37(2), 141-153.
Tiller, W. A., McCraty, R., & Atkinson, M. (1996). Cardiac coherence: a new, noninvasive measure of autonomic nervous system order. Altern Ther Health Med, 2(1), 52-65.
Tsuji, H., Larson, M. G., Venditti, F. J., Jr., Manders, E. S., Evans, J. C., Feldman, C. L., & Levy, D. (1996). Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation, 94(11), 2850-2855.
Tsuji, H., Venditti, F. J., Jr., Manders, E. S., Evans, J. C., Larson, M. G., Feldman, C. L., & Levy, D. (1994). Reduced heart rate variability and mortality risk in an elderly cohort. The Framingham Heart Study. Circulation, 90(2), 878-883.
Umetani, K., Singer, D. H., McCraty, R., & Atkinson, M. (1998). Twenty-four hour time domain heart rate variability and heart rate: relations to age and gender over nine decades. J Am Coll Cardiol, 31(3), 593-601.
van den Berg, M., Rijnbeek, P., Niemeijer, M., Hofman, A., van Herpen, G., Bots, M., . . . Stricker, B. (2018). Normal Values of Corrected Heart-Rate Variability in 10-Second Electrocardiograms for All Ages. Front Physiol, 9, 424.
Vinik, A. I., Maser, R. E., Mitchell, B. D., & Freeman, R. (2003). Diabetic autonomic neuropathy. Diabetes Care, 26(5), 1553-1579.
Watkins, P. J., & MacKay, J., .D (1980). Cardiac denervation in diabetic neuropathy. Ann Intern Med, 92(2_Part_2), 304-307.
Wolf, M. M., Varigos, G. A., Hunt, D., & Sloman, J. G. (1978). Sinus arrhythmia in acute mycardial infarction. Medical Journal of Australia, 2, 52-53.
Ziegler, D., Laux, G., Dannehl, K., Spüler, M., Mühlen, H., Mayer, P., & Gries, F. (1992). Assessment of cardiovascular autonomic function: age‐related normal ranges and reproducibility of spectral analysis, vector analysis, and standard tests of heart rate variation and blood pressure responses. Diabetic Medicine, 9(2), 166-175.
Zohar, A., Cloninger, R., & McCraty, R. (2013). Personality and Heart Rate Variability: Exploring Pathways from Personality to Cardiac Coherence and Health. Open Journal of Social Sciences, 1(6), 32-39.
